Research in the Feinman Lab
Gut-protective and regenerative strategies in graft-versus-host-disease
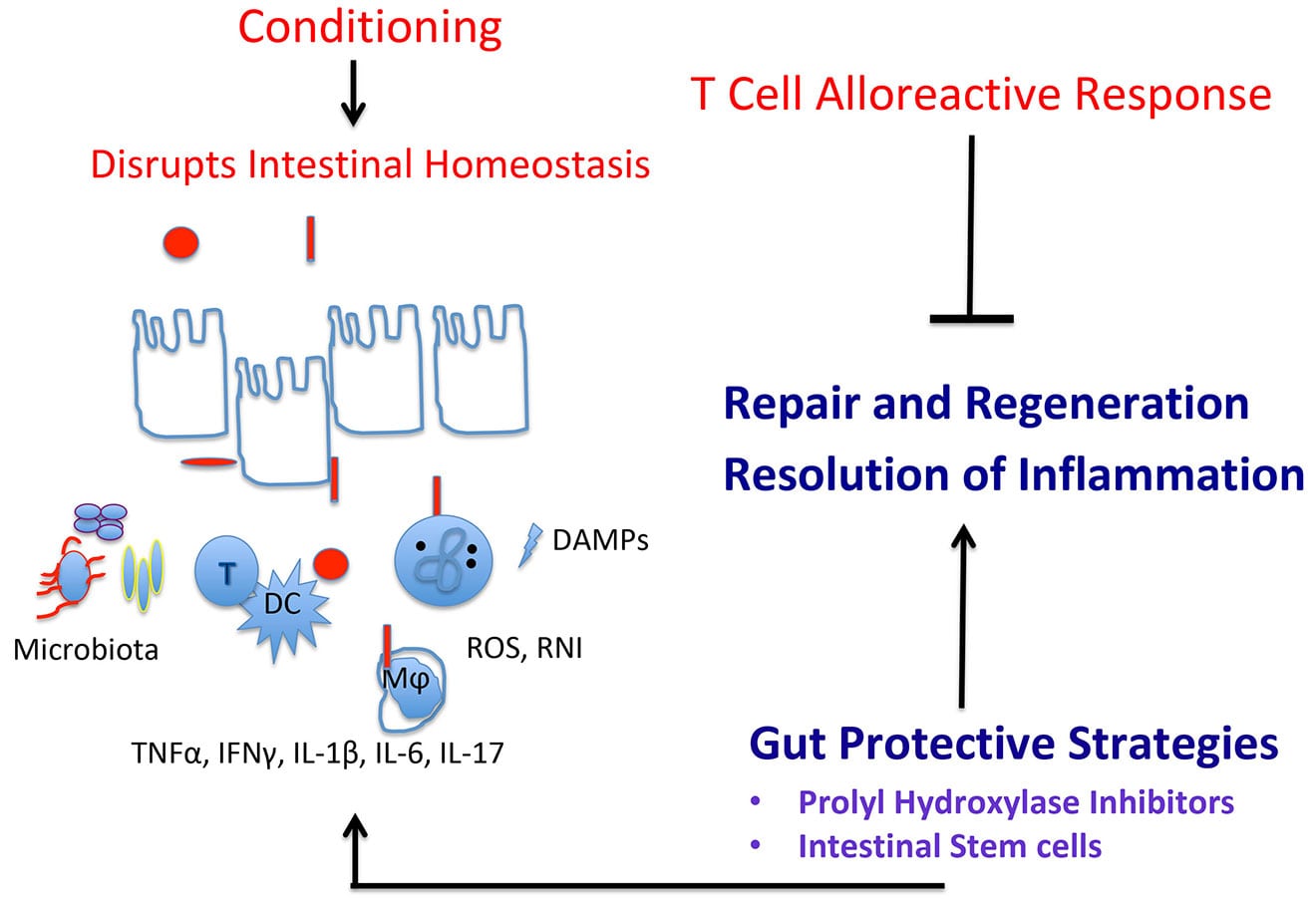
In 2013, our lab began developing a research program at Hackensack University Medical Center that identifies gut-protective strategies in graft-versus-host-disease (GVHD) which can be translated in the clinic. Having expertise in the biology of hypoxia-inducible factors (HIF) and intestinal ischemia reperfusion injury and systemic inflammatory responses, we investigated whether HIF-1 and HIF-2 played a gut-protective role in limiting conditioning- and alloreactive T cell-induced local and systemic tissue damage. Using conditional intestinal epithelial hypoxia-inducible factors HIF-1α and HIF-2α knockout mice has shown that epithelial HIF-1 and HIF-2 protect the intestinal stem cell niche from GVHD-induced injury and promote intestinal regeneration in response to gut GVHD.
To translate these studies, our lab has begun to determine whether the early short-term pharmacologic activation of HIF-1 and HIF-2 by prolyl hydroxylase inhibitors will repair conditioning-induced gut damage and mitigate T-cell alloreactivity in mouse allogeneic BMT models. Since we believe that the inability of the gut to regenerate drives the mucosal and systemic deleterious effects of GVHD, we are also testing the hypothesis that transplantation of a BMT recipient with intestinal stem cells from a related donor will repair conditioning-induced gut damage and mitigate GVHD severity. In collaboration with members at Georgetown Lombardi Comprehensive Cancer Center, our results suggest that treatment with intestinal organoids engraft in the colon and mitigate the early stages of acute GVHD.
Feinman R, Deitch EA, Watkins AC, Abungu B, Colorado I, Kannan KB, Sheth S, Caputo FJ, Lu Q, Ramanathan M, Attan S, Badami CJ, Doucet D, Barlos D, Bosch-Marce M, Semenza GL, Xu DZ. HIF-1 mediates pathogenic inflammatory responses to intestinal ischemia reperfusion injury. 2010. Am J Physiol Gastrointest Liver Physiol. 299(4):G833-843. PMID:20689059.
Kannan KB, Colorado I, Reino D, Palange D, Lu Q, Qin X, Abungu B, Watkins A, Caputo FJ, Xu DZ, Semenza GL, Deitch EA, Feinman R. Hypoxia-inducible factor plays a gut-injurious role in intestinal ischemia reperfusion injury. 2011. Am J Physiol Gastrointest Liver Physiol. 300(5):G853-861. PMID:21183660.
Oral Presentation at 56th ASH Annual Meeting, Dec 2014. Feinman R, Colorado I, Zilberberg J, Sreedhar A, Dziopa E, Dzipoa L, Yang Z and Korngold R. Intestinal Epithelial HIF-1 Plays a Protective Role in Gut Graft Versus Host Disease. Blood 2014, 124:539
Zilberberg J, Feinman R, Korngold R. 2014. Strategies for the Identification of T Cell Recognized Tumor Antigens in Hematological Malignancies for Improved Graft-versus-Tumor Responses Following Allogeneic Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 21(6): 1000-1007. PMID:254559643
Poster Presentation at 58th ASH Annual Meeting, Dec 2016. Feinman R, Colorado I, Wang K, Dzopia E, Flynn MA, Peters K, Pecora AL and Korngold R. Inhibition of HIF Prolyl Hydroxylases Mitigate Gut Graft-Versus-Host Disease. Blood 2016, 128:3349
Impact of the gut microbiome on immunotherapeutic responses in high-risk multiple myeloma
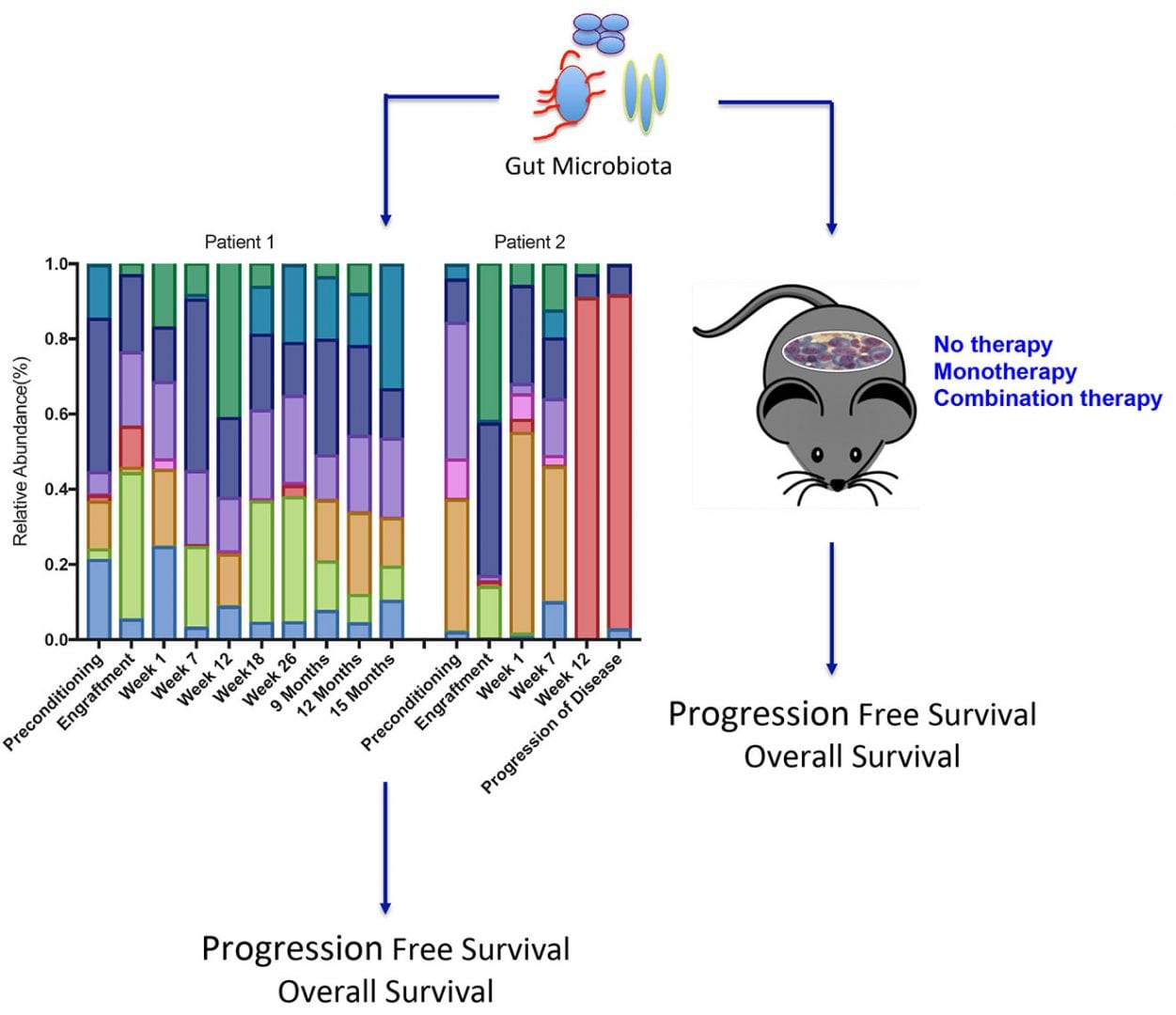
Given our longstanding research in mucosal immunology and biology of MM, our lab has begun investigating whether heterogeneity of progression-free survival and overall survival in high-risk MM patients to various immunotherapies is related to differences in the composition of the gut microbiome. Having established a strategic partnership with Second Genome, the Microbiome company, we have begun to profile the gut microbiome in stool samples collected from high-risk MM patients prior to ASCT (preconditioning), engraftment, and during treatment and follow-up. Because high-risk MM patients tend to relapse very quickly post-ASCT, they offer a window of opportunity to quickly establish whether an intervention will impact event-free survival, without having to resort to extremely long-term follow-up. Our translational studies are designed to identify unique microbiota-associated immune phenotypes that are prognostic and patients at risk for immune- and treatment-related adverse events and comorbidities.
We also seek to understand the impact of prophylactic and treatment-related antibiotics, steroids, and medications on microbiome composition and ultimately clinical outcome. In collaboration with members of our Cancer Prevention and Control Division at the John Theurer Cancer Center, we will evaluate the contribution of racial disparities, diet, obesity, age, gender, smoking, and other cancer-risk related behavioral factors on microbiome profiles. Proof of concept studies in a preclinical MM model will test whether distinct gut microbiota modulate tumor burden and survival in response to monotherapy and combination therapy. As an extension of results obtained from these studies, we plan to collaborate with members of academia and pharma to design adjuvants, such as metabolites or probiotics, from observed beneficial bacteria that promote tumor immunosurveillance and alleviate T-cell exhaustion in our preclinical MM model. We hope that the knowledge gained from our studies will have far reaching implications in predicting treatment responses for MM patients and the personalization of therapy to prevent measurable disease, minimize the duration of therapy, improve quality of life, and reduce cost of treatment.
Second Genome collaboration: press release
Impact of the gut microbiome on neoadjuvant chemotherapeutic responses in triple-negative breast cancer (TNBC)
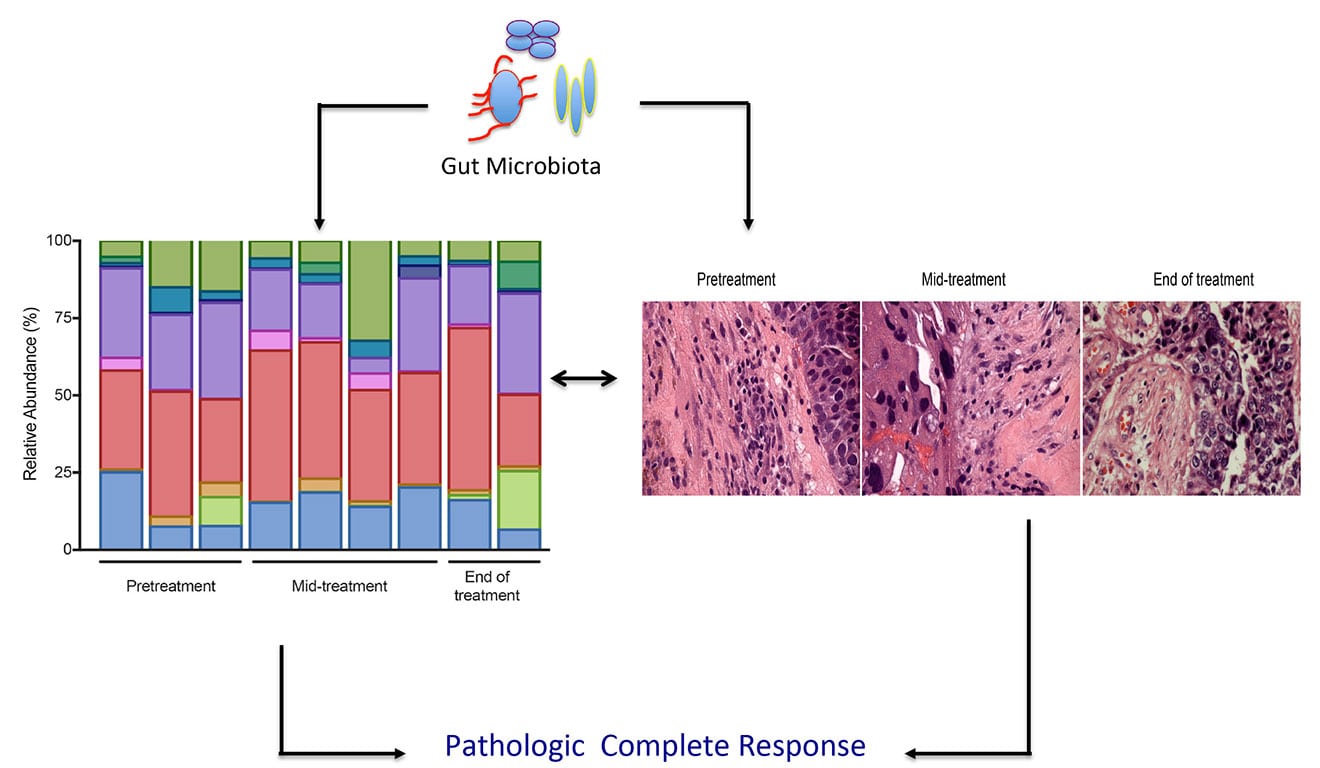
Given that only 30% of TNBC patients achieve pathologic complete response (pCR) to anthracycline-taxane-based neoadjuvant chemotherapy (NAC) and the majority of patients progress to metastatic disease, there is a need to find better therapeutic options for this patient population. Our lab has partnered with Dr. Leslie Montgomery, Chief of the Division of Breast Surgery at Hackensack University Medical Center and Co-Director of the Breast Division at John Theurer Cancer Center, to identify predictive microbiome-associated biomarkers that correlate with the likelihood of achieving pCR to standard of care neoadjuvant chemotherapy (NAC) in newly diagnosed TNBC patients. We have begun to serially profile the composition of the gut microbiome, at diagnosis, during NAC and at end of NAC. Given the prognostic significance of tumor-infiltrating lymphocytes in TNBC, studies are underway to determine if distinct microbiota signatures associated with improved pCR correlate with the activation of effector T cells in the tumor microenvironment. The impact of host genetics, epigenetics, medications, and lifestyle and environmental factors on shaping microbiome profiles, immune fitness, and outcomes will be studied extensively. Knowledge gained by understanding the mutual relationship between the gut microbiome and a patient’s immune response will help identify biomarkers and personalize microbial-based therapies to improve outcomes in TNBC patients.
Second Genome collaboration: press release
We thank the patients, collaborators, Breast Cancer Research Foundation, National Cancer Institute, Georgetown Lombardi Comprehensive Cancer Center, and John Theurer Cancer Center for supporting our current and past research projects.
